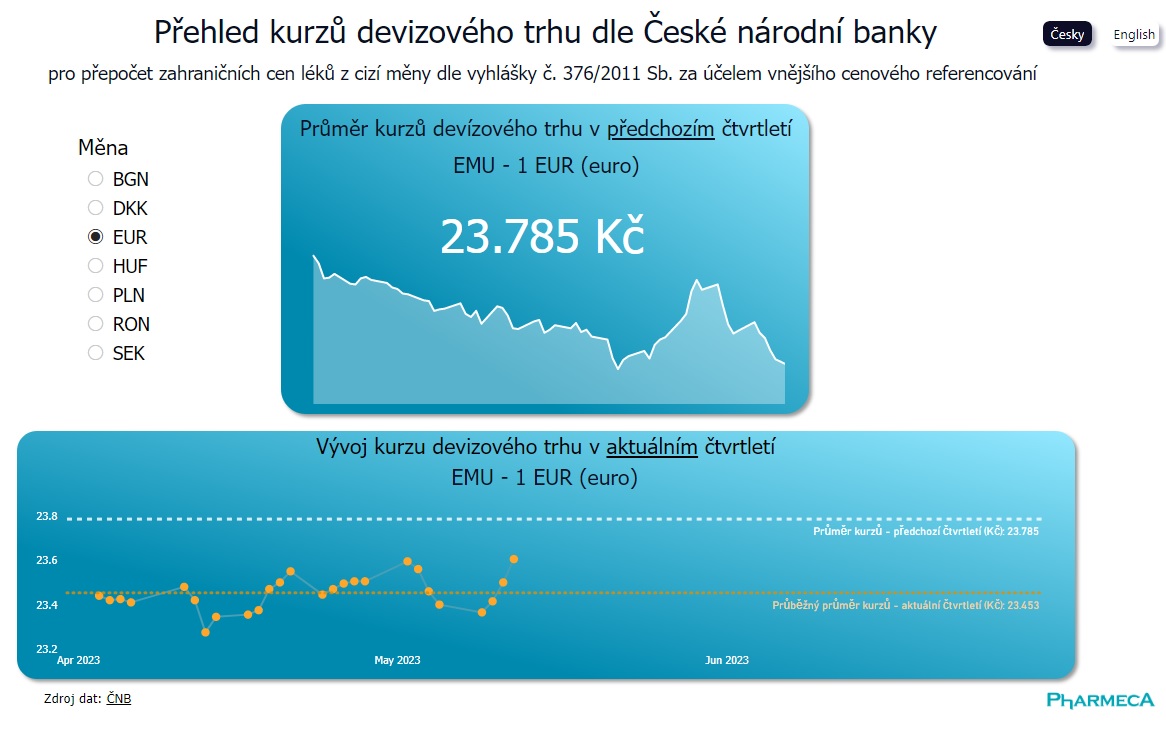
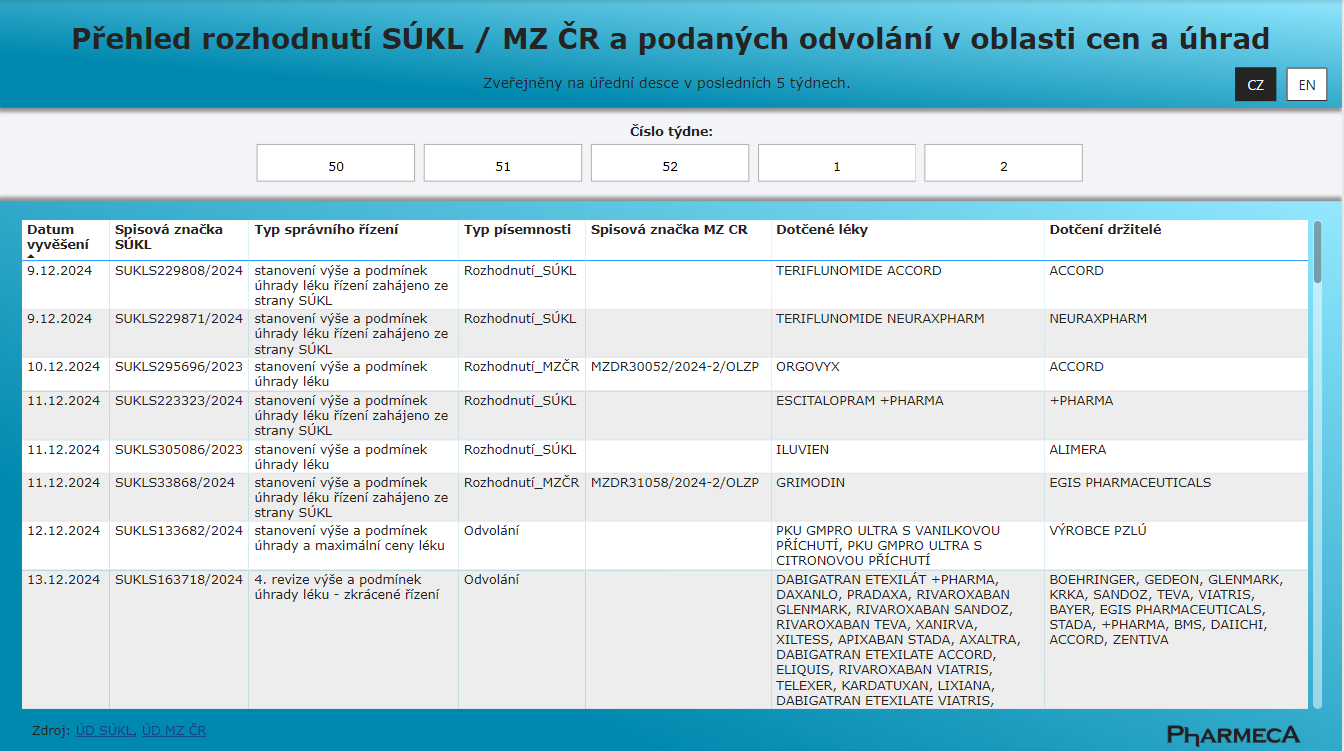
We know
the right way
Are you trying to get the price and reimbursement of your medicine or medical device, but still encounter more and more obstacles?
Are you looking for the right analysis to enforce your medicine or medical device, but keep getting lost in the data?
Are you constantly going through laws and regulations and do not know how to support your allegation?
Pharmeca, they are doctors, pharmacists, economists, lawyers, statisticians, analysts and specialists in public law.
Our work, that is the preparation of complete documents for administrative proceedings in the field of prices and reimbursement of medicines, or reimbursement of medical devices, or analysis and statistical reports for setting business strategies.
Our results are defined reimbursement in administrative proceedings, successful repeals and statements, in practise applied results of professional analyses or trained clients at in-house seminars.
It is we, who will help you achieve your goal.
Pharmeca is: