Sekce:
Data visualization
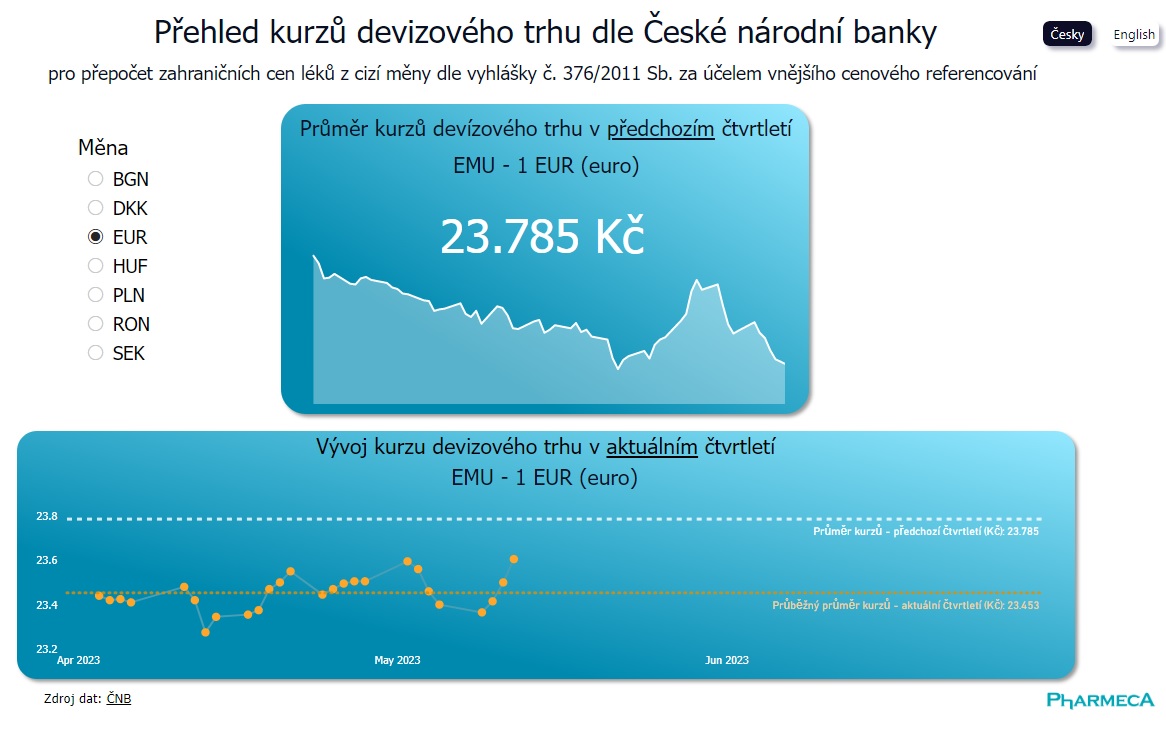
Exchange Rates for Price Referencing at a Click
The key information for conducting price referencing in administrative procedures for drug pricing and reimbursement is the average exchange rate from the previous quarter. Now, you can easily...

Pharmeca a.s.
01/28/2026
Sekce:
Data visualization
Interactive Map of Countries Used in External Price Referencing of Medicinal Products
An interactive map that visualizes the countries involved in the EPR system as applied in the Czech Republic.


Pharmeca a.s.
07/10/2025
Sekce:
Data visualization
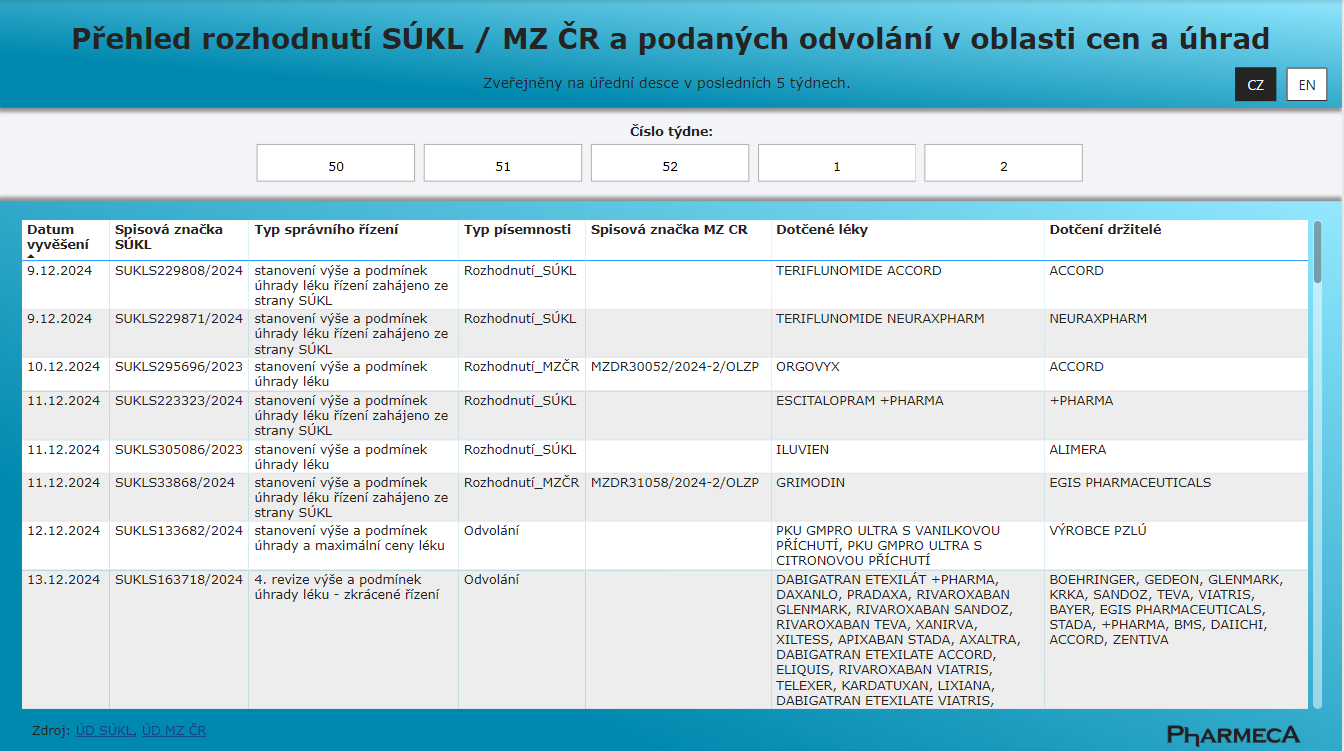
Decisions of the State Institute for Drug Control and the Ministry of Health of the Czech Republic in the Area of Pricing and Reimbursement
From January 1, 2025, Pharmeca a.s. offers an overview of SÚKL and Ministry of Health decisions on pricing and reimbursement.

Pharmeca a.s.
01/08/2025

