Roles of Key Stakeholders in the Drug Reimbursement Process in the Czech Republic
The process of determining the reimbursement of medicinal products in the Czech Republic is complex and involves several key stakeholders who collectively influence the final decision. In our recent post on decision-making practices, we highlighted the role of health insurance companies in the reimbursement process. Now, let’s take a closer look at the entities involved and how the entire mechanism works.
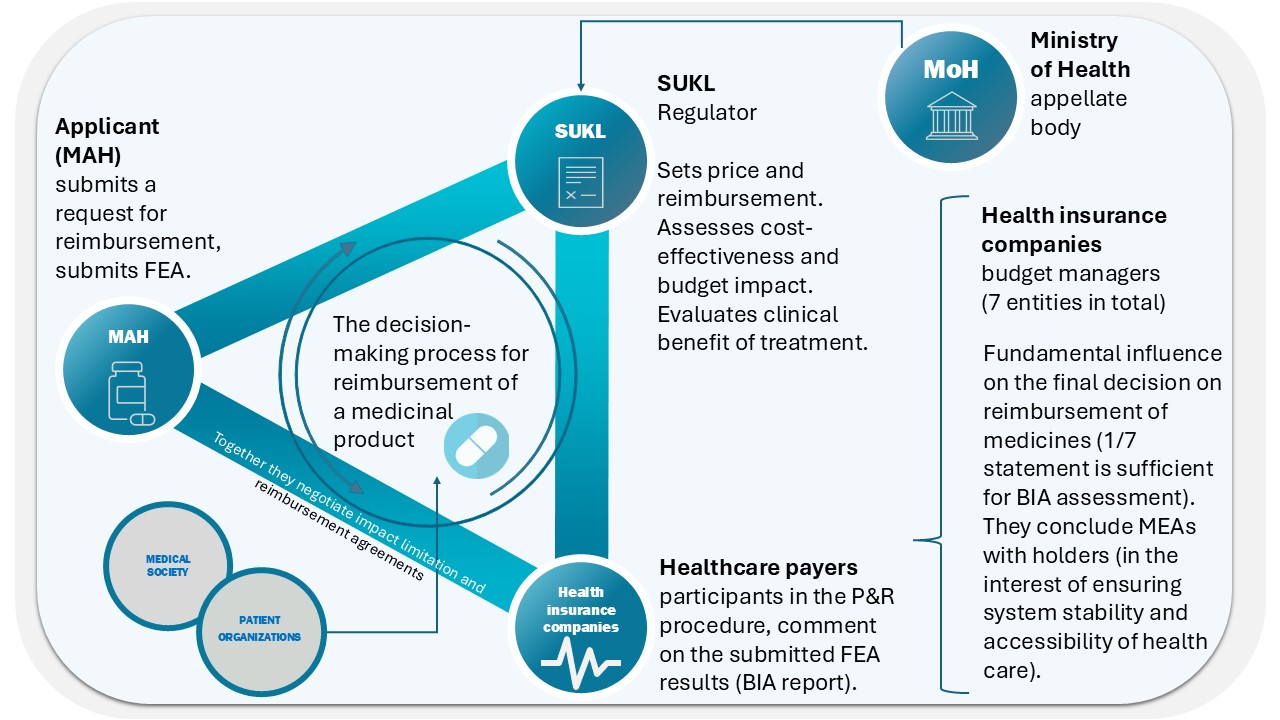
Key Stakeholders in the Reimbursement Process
- Applicant (Marketing Authorization Holder)
- Applies for reimbursement (and price) of the medicinal product.
- Provides evidence of efficacy and safety along with a pharmacoeconomic analysis that evaluates the benefits of treatment in relation to its costs (where required by law).
- Can file objections to the State Institute for Drug Control (SÚKL)’s assessment and appeal against the issued decision.
- Negotiates with payers (health insurance companies) regarding budget impact mitigation.
- State Institute for Drug Control (SÚKL)
- Acts as the regulatory body responsible for setting the price, reimbursement, and its conditions.
- Assesses cost-effectiveness and budget impact on the healthcare system.
- Evaluates the clinical benefit of the treatment.
- Ministry of Health of the Czech Republic (MoH)
- Functions as the appellate body in cases where any of the involved parties disagree with SÚKL’s decision and file an appeal.
- Healthcare Payers – Health Insurance Companies
- There are seven health insurance companies in the Czech Republic, playing a crucial role in the reimbursement process.
- They participate in proceedings alongside the applicant (in selected cases, they can also be the applicant themselves).
- Manage healthcare budgets and oversee the stability and sustainability of the system.
- Enter into reimbursement agreements with marketing authorization holders to ensure system stability and treatment availability.
- Their opinion is critical in determining reimbursement, and a positive pharmacoeconomic assessment, particularly of budget impact, serves as a basis for SÚKL’s decision. Recent decision-making practices indicate that the objection of a single health insurance company can be sufficient to deem the presented budget impact unacceptable.
- Medical Societies and Patient Organizations
- Professional societies and patient organizations are parties to reimbursement proceedings for orphan drugs. In other cases, their role is mainly supportive, SÚKL consulting them primarily on issues related to real-world clinical practice.
How Does the Decision-Making Process Work?
The entire drug reimbursement decision-making process consists of several steps:
- Application Submission – The applicant submits a reimbursement proposal along with a pharmaco-economic and budget impact analysis.
- SÚKL Assessment – The regulatory body evaluates cost-effectiveness and treatment benefits.
- Health Insurance Companies’ Opinion – They assess the economic impact and system sustainability.
- Expert (or Patient Organization) Opinions – On clinical practice (e.g., regarding real-world dosing).
- Final Decision and Right to Appeal – The Ministry of Health serves as the appellate authority.
This complex process is designed to ensure that the efficacy and safety of medicinal products are adequately financially recognized while maintaining economic sustainability within the healthcare system.

