Clinical Trials Information System (CTIS) - present and future
CTIS was established by pharmaceutical law in the Clinical Trials Regulation (Regulation (EU) No 536/2014) and will completely replace the previous system for clinical trials EudraCT (European Union Drug Regulating Authorities Clinical Trials Database) after 30th January 2025. CTIS supports interactions between clinical trial sponsors (researchers or companies that run a clinical trial and collect and analyse the data) and regulatory authorities in the EU Member States and EEA countries, throughout the lifecycle of a clinical trial.
CTIS is guaranteed by European Medicines agency (EMA) and works in a secured workspace.
The important milestones for clinical trials:
- CT starts after 30th January 2023 – the submission must be completed through CTIS
- CT ends before 30th January 2025 – CT is maintained in EudraCT or national systems
- CT is expected to continue after 30th January 2025 – transition from the EudraCT or national systems to the CTIS is necessary.
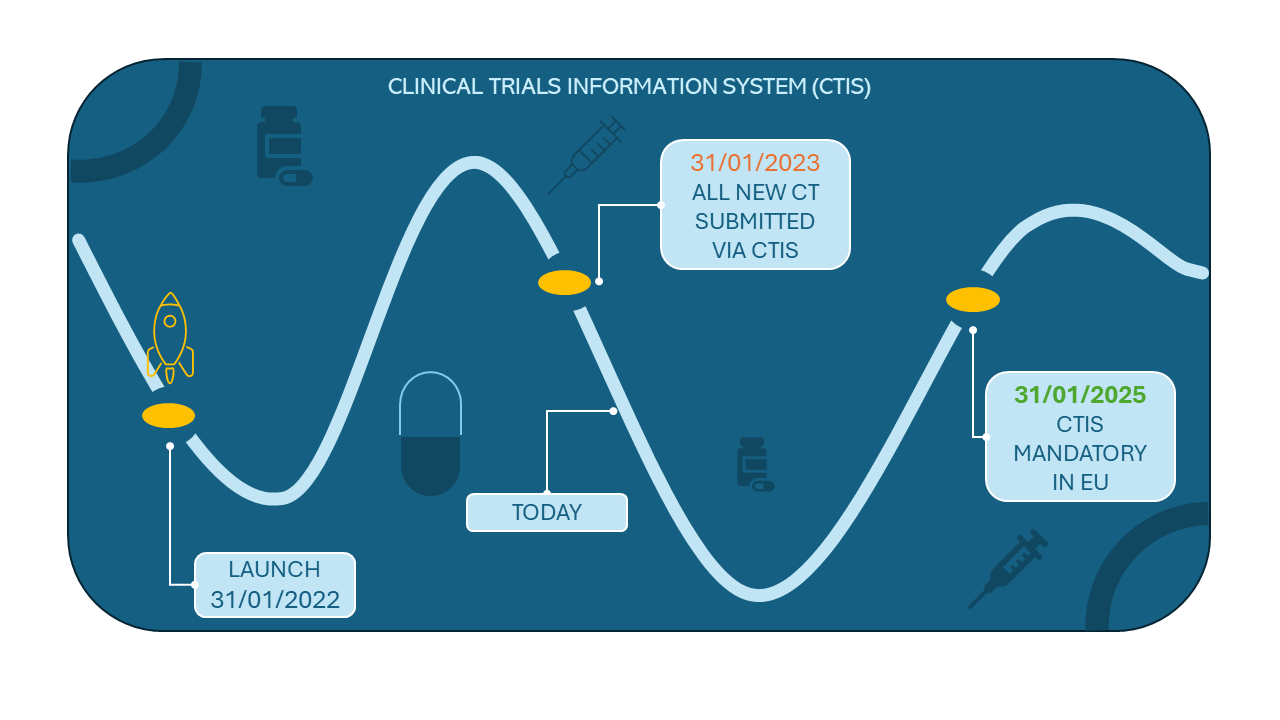
Although the deadline is still 11 months away, according to the EMA's recommendations, the transition process should begin without delay. There are a few tricky points in the transition process that are worth noting.
- The transition should take 2 months (according to the EMA methodology) but be prepared for a longer period. Once the process is complete, all information and data are processed through CTIS.
- The new country should be included in clinical trial after the transition in all current clinical trial countries is complete.
- The national language especially with special characters, can be a problem. You must provide labels in the national language.
- Relation in workspace can be unstable, in which case the submission may not complete successfully and a resession may be required.
Because the system is not intuitive and not easy to use (experience), there are guidance and Q&As from EMA (link) as well as webinars (link) for users and sponsors.
EMA has also published an interesting video to improve awareness of the regulation of the clinical trials system: https://youtu.be/0nQ2ABHa9L0?feature=shared
In the next video, EMA introduces CTIS workspaces and common system functionalities to users: https://youtu.be/NSQmVtqhmWo?feature=shared
EMA publishes CTIS statistics weekly (link). Last published data from 6th to 12th February 2024:
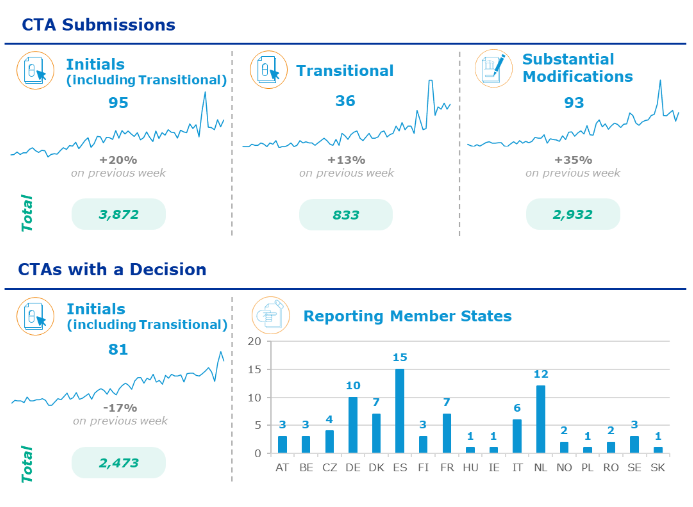
Despite difficulties, until now there are 2473 successful submissions. However, there are also many CTs that must go through the transition either.
If this is also the case with your CT, don't delay any longer and go for the submission. Good luck, you'll make it.

