European Health Data Space 2024 update
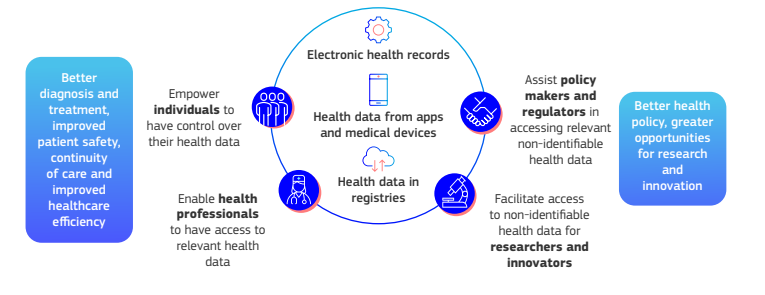
The European Health Data Space should lead to the empowerment of citizens and support for research and innovation.
EU residents will have more control over their health data, being able to access it electronically and share it across borders. This is particularly beneficial for cross-border healthcare, allowing individuals to access medical records and receive care seamlessly throughout the EU.
The EHDS promotes the secondary use of health data for research, innovation, and policy-making. Researchers and innovators will be able to access health data more easily and securely, which could drive the development of new treatments, medical devices, and AI applications.
The main objectives of the European Health Data Space (source: excerpt from the Communication from the Commission to the European Parliament and the Council: The European Health Data Space: Harnessing the power of health data for people, patients, and innovation):
EHDS - main points:
- 3 May 2022 – European Commission launched the EHDS regulation proposal to easier exchange and access health data at EU level.
- 6 December 2023 – The EU Council approved the regulation proposal.
- 15 March 2024 – After five tripartite meetings between Parliament, the Council and the Commission, so called “trialogue”, an agreement on provisional proposal was reached between the European Parliament and the EU Council. The compromised text contains e.g. a right to opt-out of the system, an electronic health records system assessment, a data localisation and some specific timelines of implementation.
- 24 April 2024 - The European Parliament formally approved EHDS regulation proposal. After the end of the legislative process, the European Parliament and the Council will adopt the final text of the new Regulation.
- The proposal is currently in its first reading procedure, document identifier COM(2022)0197. The European Parliament is expected to decide on the proposed amendments by the end of December 2024.
EHDS general information:
One key feature of the EHDS is the introduction of common standards for electronic health record (EHR) systems across the EU, fostering interoperability and creating a single market for these systems. This includes a mandatory self-assessment for systems to ensure secure data storage and transfer.
The regulation also establishes an opt-out system where individuals can choose to restrict access to their data for secondary purposes, such as research, while still allowing essential data sharing for healthcare delivery. Member States will manage access requests for health data and may implement stricter rules for sensitive information like genetic data.
The EHDS framework is expected to enhance patient care by reducing unnecessary testing and improving evidence-based decision-making. It also strengthens privacy protections while enabling the reuse of health data for public health and research purposes.

